Recombinase Polymerase Amplification: Currently, laboratory diagnosis of viral diseases mostly depends on the amplification of the genetic material and detection by real-time polymerase chain reaction (PCR) (Johnston, Pieniazek et al. 2006; Alemayehu, Feghali et al. 2013) but which is not suited for the use at the point-of-need because: 1) this requires complex devices, 2) it must be performed by a highly qualified person and 3) the reagents must be stored at -20°C.
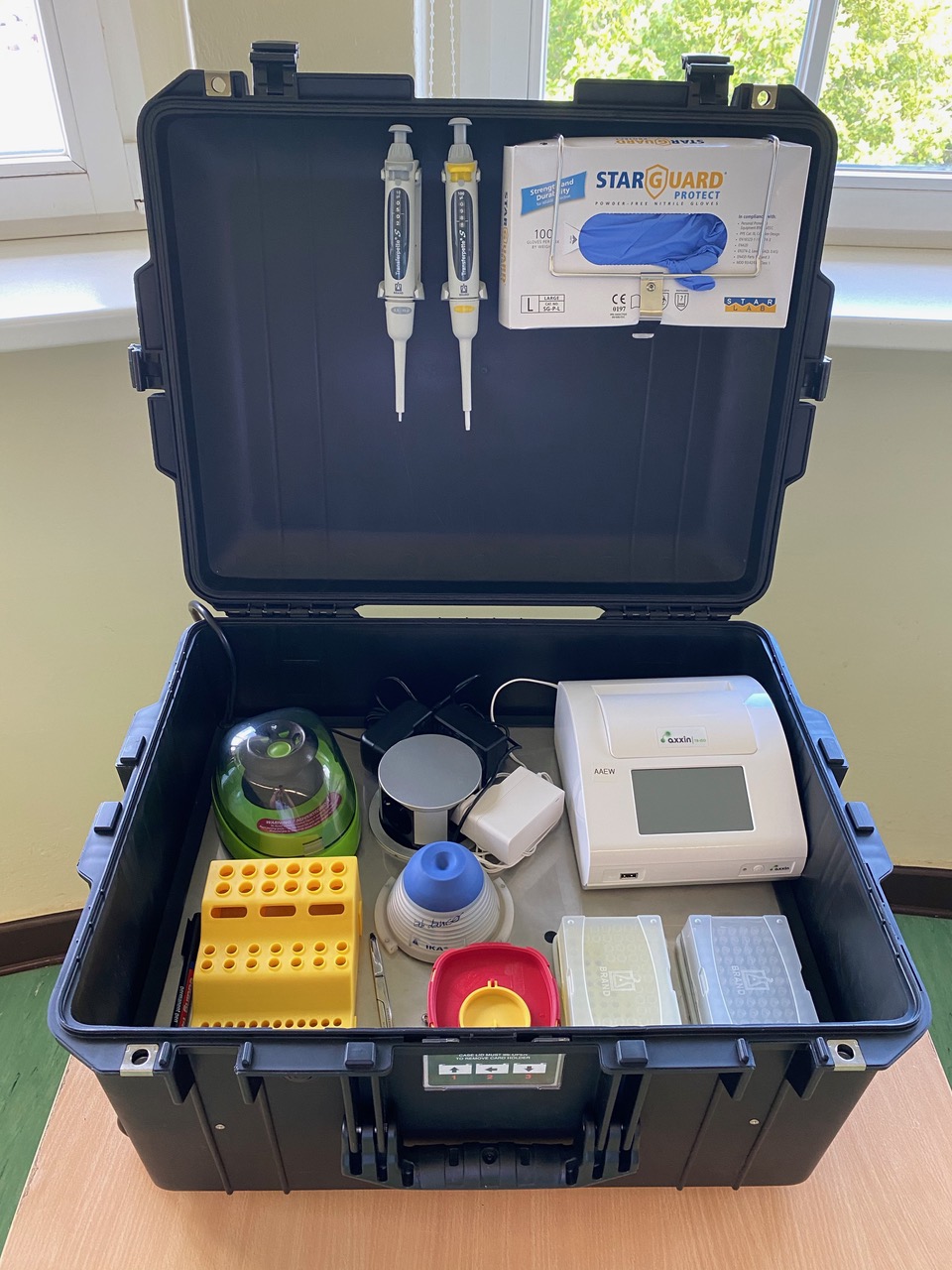
The overall objective of the proposed work is to provide a solution to introduce mobile diagnostic capabilities and capacity for infectious disease detection at point of need in partner country to improve diagnostic response of national and state teams working on outbreak management. There are 3 steps:
Optimization of a nucleic acid extraction method: A simple lysis non-purification method will be adapted. The approach will be comparable with other commercial standard methods. The effect of inhibitors on RPA performance will be studied. The best sample-to-buffer ratio for assay sensitivity will be identified.
Establishment of the RPA assays: Tasks for the development of RPA assays include sequence alignments, RPA primer and probe design, generation of a molecular standard. The assay´s sensitivity, specificity and cross-reactivity will be tested. Positive and negative controls will be included. The RPA assays will be developed for simultaneous detection of pathogens. Some of these assays have been previously developed by the Gottingen team but in this project will be adapted to the local sequences.
Validation of the RPA assays: Both the extraction procedure and the RPA assays will be evaluated using field samples. In a first step, spiked and clinical samples will be tested in the laboratory. In a second step the test will be evaluated under field conditions in a field trial. All samples will be tested simultaneously with the proposed RPA assays and real-time PCR, the current gold standard.
The mobile laboratory and point of need pathogen detection (RPA) will rapidly diagnose endemic pathogens with potential outbreak. It can be used in remote areas as it does not require highly skilled technicians and uses solar panels as the power source.